World’s First Human Trial (12 months) Success News
The technology, named SWITCH (an acronym for The Stentrode With Thought-Controlled Didgital Switch) from Synchron, which is being in the spotlight as the world’s first human trial of invasive BCI, allows patients to operate the device simply by thinking.
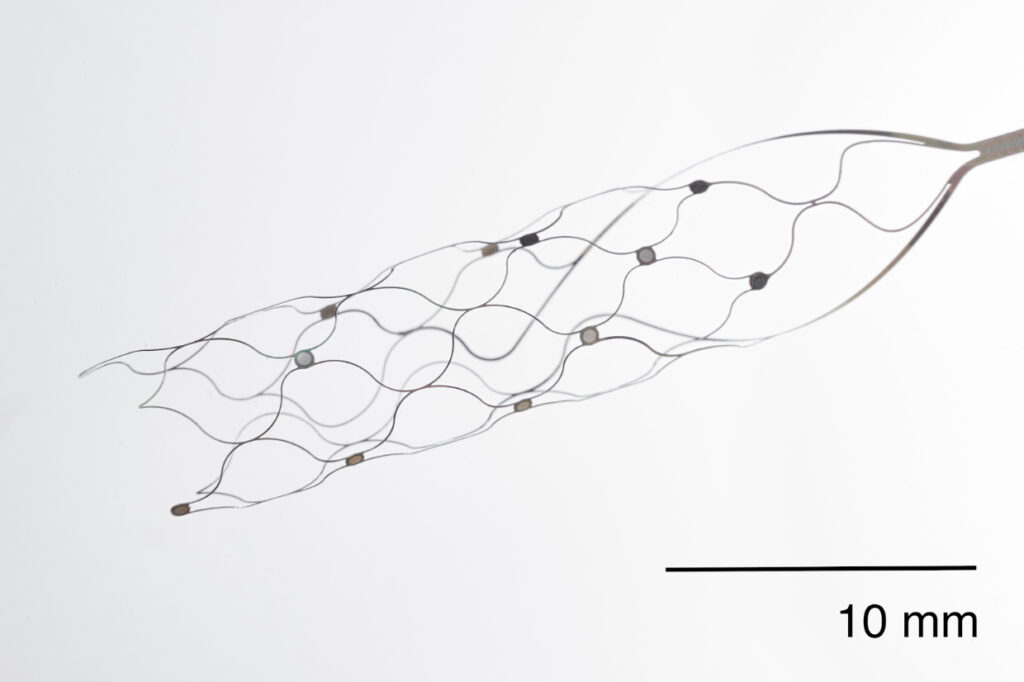
The BCI device, like a stent, can be inserted through a blood vessel into the upper part of the brain and has a digital switch inside it. According to a January 2023 press release, Synchron has successfully completed a 12-month safety test trial of four severely paralyzed patients. The details were published in JAMA (Journal of the American Medical Association). *1 During the 12 months, the four trial patients have had no adverse events and have remained safe with no vessel occlusions or device migration. This is good news for patients who have been waiting for the BCI to become commercially available. The device allows for free control of the computer and communication. The strong advantage of this device is that, unlike other implantable BCIs, it does not require surgery by a surgeon. This stented BCI is minimally invasive because it passes through blood vessels, similar to the stent procedure that is done everywhere in the world. According to the article, the number of people who could benefit from these BCI devices by 2025 is estimated to be as many as 50 million in developed countries alone.
Synchrone plans to file an application with the FDA this year.
In addition to Synchron, there are a number of other promising innovation companies in the BCI field, including Neuralink, Blackrock, BrainGate, ClearPoint, Neurable, and many others.
Reported by Nobuko Schlough, Feb. 10, 2023




